Freestanding CuS nanowalls: ionic liquid-assisted synthesis and prominent catalytic performance for the decomposition of ammonium perchlorate†
Abstract
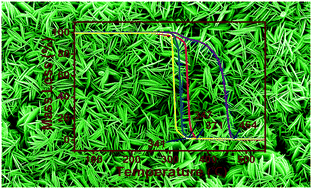
Rational design of ingenious strategies to build hierarchical architectures of nanomaterials is highly desirable and technically important due to their excellent properties and widespread application in varied fields. Here, we report a facile one-pot approach for the solvothermal synthesis of freestanding CuS nanowalls with the assistance of a common ionic liquid, 1-decyl-3-methylimidazolium bromide ([C10mim]Br). In the presence of [C10mim]Br, intriguing CuS nanowalls have been fully constructed from the as-generated CuS thin nanosheets at the liquid–liquid interface of chloroform and water. An outstanding feature is that no hard template is utilized to support and/or assist the growth of CuS nanowalls. The as-prepared CuS nanowalls have been characterized and analyzed systematically and a series of factors affecting the morphology have been investigated. It is found that the alkyl chain length, anionic nature and heterocyclic structure of ionic liquids play an important role in directing the formation of freestanding CuS nanowalls. A possible growth mechanism for the formation of CuS nanowalls is presented based on control experiments. In addition, the as-synthesized CuS nanowalls demonstrate prominent catalytic activity for the thermal decomposition of ammonium perchlorate (AP).


 Please wait while we load your content...
Please wait while we load your content...