Solvent-controlled synthesis of various Anderson-type polyoxometalate-based metal–organic complexes with excellent capacity for the chromatographic separation of dyes†
Abstract
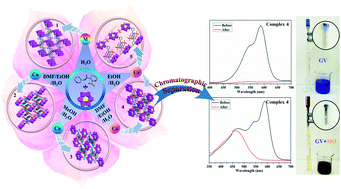
By changing solvent systems, five new Anderson-type polyoxometalates (POMs)-based metal–organic complexes, namely, {(H2PCAP′)2[CrMo6(OH)5O19]}·H2O (1), {Cu3(PCAP)2[CrMo6(OH)5O19](H2O)3(DMF)2}·4H2O·2HCHO (2), {Cu3(PCAP)2[CrMo6(OH)6O18]Cl(H2O)5}·10H2O (3), {Co3(PCAP)2[CrMo6(OH)5O19](H2O)6}·9H2O·HCHO (4) and {Co3(HPCAP)2[CrMo6(OH)6O18]2(H2O)10}·7H2O·2CH3CH2OH (5) (HPCAP = 3-(2-pyridinecarboxylic acid amido)pyridine, PCAP′ = 3-(pyridinecarboxylic acid)amido pyridine), were successfully synthesized and structurally characterized by single-crystal X-ray diffraction, IR spectra, powder X-ray diffraction (PXRD) and thermogravimetric analyses (TGA). Complex 1 was obtained with H2O as the solvent and exhibited a 3D supramolecular network based on CrMo6 anions and protonated H2PCAP′ molecules via hydrogen bonds. It should be noted that the PCAP′ was in situ transformed from HPCAP. Complexes 2–5 were synthesized with different mixed solvents. In complex 2, the CrMo6 anions acted as an inorganic bridging ligand to link the 1D [Cu3(PCAP)2]n4n+ chains to construct a 2D layer. Complexes 3 and 4 showed similar 3D frameworks. Two orientations of metal–organic loops [M2(PCAP)2] (M = Cu for 3, Co for 4) could be observed, which directly extended the 1D M–CrMo6 chains into 3D networks with a 3,3,4,4-connected {42·123·14}{42·6}2 topology. In 5, the 1D Co–CrMo6 inorganic chain, [Co2(PCAP)2] binuclear loop unit and discrete CrMo6 polyanion were connected with each other by hydrogen-bonding interactions to form a 1D ladder-like supramolecular dual chain. The structural diversities showed that the solvents play key roles in the construction of various architectures and in the in situ transformation of HPCAP. The adsorption behaviours of the title complexes for organic dyes were investigated in detail. All of the title complexes showed an efficient adsorption capacity for the cationic dyes gentian violet (GV) and methylene blue (MB). In particular, complex 4 could selectively separate GV from the mixture of GV&RhB and GV&MO within 5–10 min, which can be used as a chromatography column for dye removal. In addition, the electrochemical properties of the title complexes were also studied.


 Please wait while we load your content...
Please wait while we load your content...