Controlling the distance between hydrogen-bonded chloro-s-triazine tapes: crystal engineering using N-alkyl chains and the influence of temperature†
Abstract
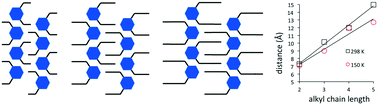
The bis-N-alkyl-chlorotriazines show a strong tendency to form one-dimensional hydrogen-bonded tapes in the solid state. We predicted that increasing the alkyl chain length in the simplest tape-forming molecule would enable a controlled increase of the inter-tape spacing in the crystalline state. A series of bis-N-alkyl groups from bis-N-ethyl to bis-N-pentyl were synthesised and structurally characterised using single-crystal and powder X-ray diffraction at low temperature and ambient temperature. At low temperature the alkyl chains are rarely linear, instead adopting a variety of conformations to achieve close packing. Despite this variation, there is still a strong correlation of alkyl chain length with the inter-tape spacing in a single direction in the crystal. This correlation is strongest at ambient temperature where the longer chains are highly disordered and extended. We observe an “odd-number carbon” effect where bis-N-propyl 1b and bis-N-pentyl 1d derivatives undergo a structural phase transition with temperature.


 Please wait while we load your content...
Please wait while we load your content...