Four coordination polymers based on dinuclear and trinuclear units with a new multifunctional pyridyl-dicarboxylate ligand: luminescence and magnetic properties†
Abstract
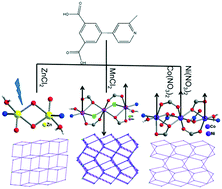
Four coordination polymers of different transition metal ions with a new pyridyl-dicarboxylate ligand, 4-(3,5-dicarboxylphenyl)-2-methylpyridine (H2L), were synthesized under solvothermal conditions and their structures and properties were characterized. They are formulated as [Zn(L)(H2O)]·H2O (1), [Mn3(L)2Cl2(H2O)2]·2H2O (2), [Co3(L)2(NO3)2(H2O)2] (3) and [Ni3(L)2(NO3)2(H2O)2]] (4). Compound 1 exhibits a 2D network with a (3,6)-connected kgd topology based on the dinuclear [Zn2O2] units. In compound 2, the linear trinuclear [Mn3(μ-Ocarboxylate)2(COO)2Cl2] units with mixed carboxylate and μ-Cl triple bridges are cross-linked by the L ligands into a 3D framework with a (3,6)-connected rtl topology. Compounds 3 and 4 are isostructural and both show 2D networks in which the linear trinuclear [M3(μ-Ocarboxylate)2(COO)2(μ-ONO3)2] units (M = Co (3) and Ni (4)) are cross-linked by the L ligands. Compound 1 shows strong blue emissions in the solid state at room temperature. Magnetic studies indicate that the mixed triple [(μ-Ocarboxylate)(COO)Cl] bridges transmit antiferromagnetic interactions between Mn(II) ions in 2, while the mixed triple (μ-Ocarboxylate)(COO)(μ-ONO3) bridges in 3 and 4 transmit ferromagnetic interactions. Further magnetic studies demonstrated that the Co(II) compound shows no long-range magnetic ordering at 2 K.


 Please wait while we load your content...
Please wait while we load your content...