Structure identification of Ti(iv) clusters in low-temperature TiO2 crystallization: creating high-surface area brush-shaped rutile TiO2†
Abstract
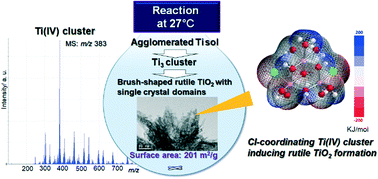
We demonstrate for the first time a controlled reaction of Cl-coordinating Ti(IV) clusters in low-temperature hydrolysis (27 °C) to create large-surface area rutile TiO2 crystals. Trinuclear Ti(IV) cluster species were produced by the partial hydrolysis of an amorphous TiO2 derived from TiCl4. The structurally optimized cluster had a pseudo-cubane structure with edge-shared Ti(IV) octahedra exhibiting unique stacked electron density units. It is postulated that breaking of Ti–OH bonds in the cluster and the intermolecular assembly of the clusters during further polycondensation reactions resulted in the formation of corner-shared Ti(IV) octahedra. The controlled stability of the Ti(IV) clusters owing to reaction solvents enables the creation of rutile TiO2 crystals with intertwined brush-shaped TiO2 single crystal domains, with a large surface area of up to 201 m2 g−1. Understanding low-temperature reactions of transition-metal cluster intermediates would benefit the design criteria of many transition-metal oxide crystals for versatile applications.


 Please wait while we load your content...
Please wait while we load your content...