Study of molecular interactions and chemical reactivity of the nitrofurantoin–3-aminobenzoic acid cocrystal using quantum chemical and spectroscopic (IR, Raman, 13C SS-NMR) approaches†
Abstract
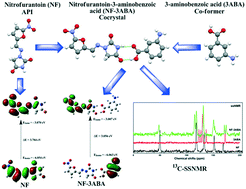
Investigations of structural reactivity, molecular interactions and vibrational characterization of pharmaceutical drugs are helpful in understanding their behaviour. The aim of this study is to determine the molecular, electronic and chemical properties of the antibiotic drug nitrofurantoin (NF), after cocrystallisation with 3-aminobenzoic acid (3ABA) and to understand how those changes lead to variation of properties in the cocrystal NF–3ABA. NF–3ABA formation is explained by stabilization via the hydrogen-bond network between NF and 3ABA molecules. It is thoroughly characterized by IR, Raman and CP-MAS solid-state 13C NMR techniques, along with quantum chemical calculations. The results of IR, Raman, and 13C NMR analyses showed that imide N–H23 and C12![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of NF interact with the acid C
O of NF interact with the acid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –OH groups in 3-ABA, respectively. Therefore the IR, Raman, and 13C NMR spectra verified the formation of N–H⋯O and O–H⋯O hydrogen bonds. To study hydrogen bonding interactions theoretically in NF–3ABA, two functionals B3LYP and wB97X-D have been used. A comparison is made between the results obtained by B3LYP and those predicted at the wB97X-D level. It is found that wB97X-D is best applied density functional theory (DFT) functional to describe the hydrogen bonding interactions. The strength and nature of hydrogen bonding in NF–3ABA have been analysed by quantum theory of atoms in molecules (QTAIM) and natural bond orbital (NBO) analysis. To validate the results obtained by QTAIM theory and to study the long-range forces, such as van der Waals interactions, the steric effects in NF–3ABA, the reduced density gradient (RDG) and the isosurface have been plotted using Multiwfn software. QTAIM and isosurface analysis suggested that the hydrogen bonding interactions present in NF–3ABA are moderate in nature. The calculated HOMO–LUMO energy gap shows that NF–3ABA is more active than NF and 3ABA. Chemical reactivity descriptors are calculated to understand the various aspects of pharmacological sciences. Chemical reactivity parameters show that NF–3ABA is softer and chemically more reactive than NF. The results suggest that cocrystals can be a feasible alternative for positively changing the targeted physicochemical properties of an active pharmaceutical ingredient (API).
O and –OH groups in 3-ABA, respectively. Therefore the IR, Raman, and 13C NMR spectra verified the formation of N–H⋯O and O–H⋯O hydrogen bonds. To study hydrogen bonding interactions theoretically in NF–3ABA, two functionals B3LYP and wB97X-D have been used. A comparison is made between the results obtained by B3LYP and those predicted at the wB97X-D level. It is found that wB97X-D is best applied density functional theory (DFT) functional to describe the hydrogen bonding interactions. The strength and nature of hydrogen bonding in NF–3ABA have been analysed by quantum theory of atoms in molecules (QTAIM) and natural bond orbital (NBO) analysis. To validate the results obtained by QTAIM theory and to study the long-range forces, such as van der Waals interactions, the steric effects in NF–3ABA, the reduced density gradient (RDG) and the isosurface have been plotted using Multiwfn software. QTAIM and isosurface analysis suggested that the hydrogen bonding interactions present in NF–3ABA are moderate in nature. The calculated HOMO–LUMO energy gap shows that NF–3ABA is more active than NF and 3ABA. Chemical reactivity descriptors are calculated to understand the various aspects of pharmacological sciences. Chemical reactivity parameters show that NF–3ABA is softer and chemically more reactive than NF. The results suggest that cocrystals can be a feasible alternative for positively changing the targeted physicochemical properties of an active pharmaceutical ingredient (API).


 Please wait while we load your content...
Please wait while we load your content...