sp2CH⋯Cl hydrogen bond in the conformational polymorphism of 4-chloro-phenylanthranilic acid†
Abstract
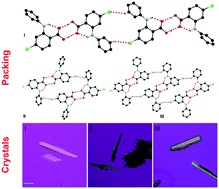
Chlorine can participate in numerous interactions such as halogen bonding, hydrogen bonding, and London dispersion in the solid state. In this work, we report the influence of a chlorine substituent on the polymorphism of a potential anticancer drug, 4-chloro-phenylanthranilic acid (CPAA). Three polymorphs have been discovered for this compound, and the three forms were characterized by single-crystal X-ray diffraction, power X-ray diffraction (PXRD), FT-IR, and Raman spectroscopy. Both conformational flexibility of the molecule and the sp2CH⋯Cl hydrogen bond seem to lead to the polymorphism of the system. The phase behavior was investigated by differential scanning calorimetry (DSC), with the conclusion that form II converts to III upon heating. A conformational scan shows the conformational minima corresponds to the conformers existing in the polymorphs. Lattice energy calculations show energies of −106.70, −104.72, and −194.42 kJ mol−1 for forms I to III, providing information on relative stability for each form. Hirshfeld analysis revealed that intermolecular interactions such as H⋯H, C⋯H, H⋯Cl, and H⋯O contribute to the stability of the crystal forms.


 Please wait while we load your content...
Please wait while we load your content...