Selective separation of aqueous sulphate anions via crystallization of sulphate–water clusters†
Abstract
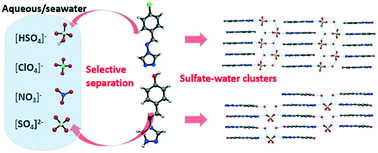
In this study, the potential of oxoanion separation with the ligands N-4-methylbenzyl-4-amino-1,2,4-triazole (L1), N-4-fluorobenzyl-4-amino-1,2,4-triazole (L2), N-4-hydroxy benzyl-4-amino-1,2,4-triazole (L3), and N-3-methoxy-4-hydroxybenzyl-4-amino-1,2,4-triazole (L4) was explored. The salts of sulphate, nitrate, and perchlorate were crystallized in the form of [HL1]+[HSO4]−·H2O, [HL2]+[HSO4]−·H2O, 2[HL3]+[SO4]2−·2(H2O), and 2[HL4]+[SO4]2−·H2O, [HL3]+[NO3]−·H2O and [HL4]+[NO3]−, and [HL3]+[ClO4]− and [HL4]+[ClO4]−, respectively. Competitive crystallization and competitive sulphate-binding experiments demonstrated special recognition of the sulphate–water clusters (in the forms of [HSO4−·H2O]n or [SO42−·H2O]n) via the parallel packed 3-D architectures of HL1+, HL2+, and HL4+ cations, which were far more effective and selective than the non-3-D structure of HL3+ cation. The observed selectivity for sulphate, nitrate, and perchlorate was found to be anti-Hofmeister. In addition, the selective separation of sulphate anions in seawater was investigated, which demonstrated the real-world application of the present strategy.


 Please wait while we load your content...
Please wait while we load your content...