Abstract
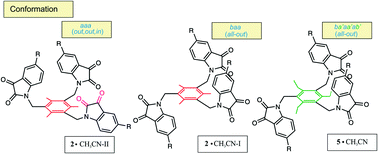
Single crystal X-ray diffraction allowed the identification and characterization of new solvates of tripodal trimethylbenzene- and triethylbenzene-based compounds bearing 1H-indole-2,3-dione (isatin) groups. Both unsubstituted and methyl or methoxy substituted isatins were used as building blocks for the construction of the host molecules 1–6. Four different conformations of 1–6 have been observed in the crystal structures, which differ not only in the position of the heterocyclic side arms (above or below the central benzene ring) but also in the orientation of the carbonyl groups of the isatin units. In the case of the trimethylbenzene derivative 2, single crystal X-ray diffraction allowed the identification and characterization of three solvates (2a, 2b and 2c), two of which represent the polymorphic forms 2·CH3CN-I (2a, baa/all-out) and 2·CH3CN-II (2b, aaa/out,out,in). The crystal packings of 1–6 are characterized by the presence of C–H⋯O, C–H⋯N, C–H⋯π, π⋯π and CO⋯π interactions as well as Cl⋯Cl contacts in the case of the chloroform-containing solvates.


 Please wait while we load your content...
Please wait while we load your content...