A combined crystallographic and theoretical study of weak intermolecular interactions in crystalline squaric acid esters and amides†
Abstract
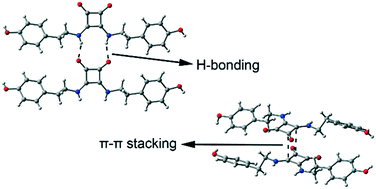
We report the synthesis and X-ray solid state structures of five squaric acid derivatives, i.e. a zwitterionic compound, namely 3-hydroxy-4-(2-pyridin-2-yl-ethylamino)cyclobut-3-ene-1,2-dione (1), a squaramide monoester, 3-ethoxy-4-(2-pyridin-2-yl-ethylamino)cyclobut-3-ene-1,2-dione (2), two differently solvated (EtOH and DMSO/water) disquaramides 3,4-bis((4-hydroxyphenethyl)amino)cyclobut-3-ene-1,2-dione (3 and 4, respectively), and a mixed hydrogen squarate and disquarate 2-(2-aminoethyl)pyridinium salt (5). All compounds form interesting supramolecular assemblies in the solid state that have been analyzed using high level DFT calculations and Bader's theory of “atoms-in-molecules”. An intricate combination of ion-pair and H-bonding interactions along with π–π stacking and anion–π contacts of the cyclobutenedione rings is crucial for the formation of the supramolecular assemblies in the solid state.


 Please wait while we load your content...
Please wait while we load your content...