Four new coordination polymers based on a pyridinetetracarboxylate ligand: syntheses, structures and high CO2/CH4 separation†
Abstract
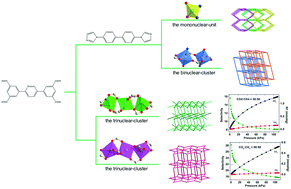
Four new coordination polymers, [Zn(TPTA)0.5(BIBP)] (1), [Mn(TPTA)0.5(BIBP)0.5(DMF)] (2), [Zn1.5(TPTA)]·DMF·H2O (3), and [Cd1.5(TPTA)]·DMF·2H2O (4) (H4TPTA = 5,5′-(pyridine-2,5-diyl)diisophthalic acid, BIBP = 4,4′-bis(imidazol-1-yl)biphenyl), have been synthesized under solvothermal conditions and fully characterized. Single-crystal X-ray diffraction analysis revealed that complex 1 is a three-dimensional (3D) 3-fold interpenetrating architecture with (4·64·8)2(42·62·82) topology. Complex 2 possesses a 3D 2-fold interpenetrating framework based on binuclear Mn2 units with (412·63) topology. Both complexes 3 and 4 exhibit trinuclear 3D non-interpenetrating (4,8)-connected networks with (46)2(412·612·84) topology. The fluorescence properties of 1, 3 and 4 have been also measured. Importantly, gas adsorption studies for 1–4 show highly selective adsorption of CO2 over CH4.


 Please wait while we load your content...
Please wait while we load your content...