Solvent effects and its role in quantitatively manipulating the crystal growth: benzoic acid as case study†
Abstract
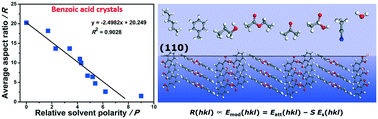
In the pharmaceutical industry, solvent effect is a fundamental and profound factor in controlling the crystal morphology and leading to different physical and chemical properties of raw materials. There is a requirement for quantitative understanding of the crystal habit modification controlled by solvents for the manipulation of crystal shapes and aspect ratios during crystal growth processes. Herein, the solvent effects on the crystal growth of benzoic acid (BA) were studied through morphology analysis of observed crystals, in conjunction with molecular dynamic (MD) simulation. The aspect ratio of experimental crystals of BA in 16 selected solvents was found to decrease on increasing the solvent polarity. Moreover, the aspect ratio changes due to molecular size of the solvents were proposed and discussed in detail. Through agreement with MD simulation, we accurately reproduced the experimental crystal morphology and the aspect ratio in alcohols. This study helps to gain quantitative understanding of the role played by solvents on the aspect ratio and morphological changes of BA crystals.


 Please wait while we load your content...
Please wait while we load your content...