Syntheses, crystal structures and characterization of three alkaline metal borates†
Abstract
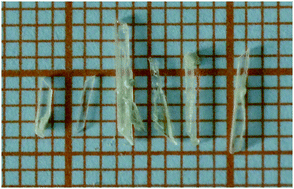
Three alkaline metal borates Cs2B7O9(OH)5, K2B4O5(OH)4·H2O, and K2B4O5(OH)4·3.6H2O have been synthesized by a hydrothermal method. They were structurally characterized by single crystal X-ray diffraction. In the three compounds, the B–O anionic groups are all isolated, which are combined through the Cs/K–O polyhedra to form a three-dimensional (3D) network. The cationic framework presents different dimensions in Cs2B7O9(OH)5, K2B4O5(OH)4·H2O and K2B4O5(OH)4·3.6H2O. For Cs2B7O9(OH)5, which has been reported before, we obtained its large-scale crystal and the UV-vis diffuse reflectance spectral data of Cs2B7O9(OH)5 indicate that it has a UV cut-off edge of 209 nm which corresponds to an energy gap of 5.93 eV. Theoretical calculations were carried out to evaluate the band gaps of the title compounds, which illuminate that the replacement of K enlarges the band gap. Besides, the structural discrepancy among Cs2B7O9(OH)5, K2B4O5(OH)4·H2O and K2B4O5(OH)4·3.6H2O is compared to analyze the effect of the cation number on the polymerization degree of the B–O anionic groups. Density functional theory calculations were employed to evaluate the band structures and the density of states suggest that Cs2B7O9(OH)5 and K2B4O5(OH)4·H2O possess wide band gaps of 5.23 and 5.24 eV, respectively. Meanwhile, the large-scale crystal of Cs2B7O9(OH)5 was obtained.


 Please wait while we load your content...
Please wait while we load your content...