Diiodomethane as a halogen bond donor toward metal-bound halides†
Abstract
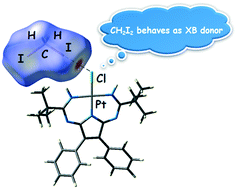
A 1,3,5,7,9-pentaazanona-1,3,6,8-tetraenate (PANT) chloride platinum(II) complex (1) was obtained via the metal-mediated double coupling of 2,3-diphenylmaleimidine with both nitrile ligands in trans-[PtCl2(NCtBu)2]. Compound 1 was then co-crystallized with diiodomethane forming solvate 1·½CH2I2. The XRD experiment reveals that this solvate displays the halogen bonds H2C(I)–I⋯Cl–Pt and hydrogen bonds I2C(H)–H⋯Cl–Pt, which join two complex and one CH2I2 molecules in a heterotrimeric supramolecular cluster. Inspection of the CCDC database reveals only one example of the halogen bond H2C(I)–I⋯I–Pt between the CH2I2 molecule and metal-coordinated halide in the structure of VEMWOA. In VEMWOA, CH2I2 serves solely as a halogen bond donor with no hydrogen bond contribution. Results of the Hirshfeld surface analysis and DFT calculations (M06/DZP-DKH level of theory) followed by topological analysis of the electron density distribution within the formalism of Bader's theory (QTAIM method) for both 1·½CH2I2 and VEMWOA confirmed the formation of these weak interactions. The evaluated energies of halogen bonds involving CH2I2 are in the 2.2–2.8 kcal mol−1 range.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...