Spin pairing, electrostatic and dipolar interactions influence stacking of radical anions in alkali salts of 4,5-dichloro-3,6-dioxocyclohexa-1,4-diene-1,2-dicarbonitrile (DDQ)†
Abstract
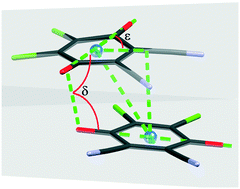
A series of five novel air-stable alkali salts of the 4,5-dichloro-3,6-dioxocyclohexa-1,4-diene-1,2-dicarbonitrile radical anion (DDQ) were prepared and structurally characterised. Four of them (Li, Na and two Cs salts) involve Peierls-distorted π-stacks of radicals, while the K salt-structure comprises equidistant stacks of radicals. Different orientations of radicals in the stacks and different directions of the offset have been observed. For an unambiguous description of the π-stacking geometry of quinones and semiquinone radicals, the geometric parameters calculated by the program PLATON are not sufficient. Therefore, two additional geometric parameters are introduced: δ (torsion angle between molecular axes O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C⋯C
C⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of contiguous rings) and ε (angle between the molecular axis O
O of contiguous rings) and ε (angle between the molecular axis O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C⋯C
C⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and direction of the offset). On the basis of these geometric parameters, the optimal stacking orientations can be quantified and correlated with the polarity and steric effects.
O and direction of the offset). On the basis of these geometric parameters, the optimal stacking orientations can be quantified and correlated with the polarity and steric effects.


 Please wait while we load your content...
Please wait while we load your content...