Synthesis of steroidal molecular compasses: exploration of the controlled assembly of solid organic materials†
Abstract
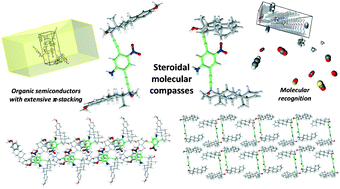
In this work, five steroid-flanked derivatives of p-nitroaniline were studied to assess the influence of these rigid, chiral frameworks on the solid-state patterning of a dipolar dye, striving to drive the self-assembly of noncentrosymmetric crystals, which is of interest in the development of organic materials for nonlinear optics and piezoelectricity. The featured compounds were prepared using Sonogashira cross-coupling reaction with 17α-ethynyl steroids as a single key step. The structures of these new steroidal derivatives were established using one and two dimensional NMR 1H and 13C experiments. Single X-ray diffraction analysis unequivocally confirmed the presence of the steroidal molecular compasses 8 and 11, whose potential applicability for organic materials is preliminarily assessed via computational modeling, finding candidates for nonlinear optics, organic electronic materials and molecular recognition.


 Please wait while we load your content...
Please wait while we load your content...