Size-controlled synthesis and electrochemical performance of porous Fe2O3/SnO2 nanocubes as an anode material for lithium ion batteries†
Abstract
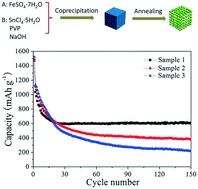
Uniform and monodisperse porous Fe2O3/SnO2 nanocubes with different particle sizes were synthesized by a template-free, economical aqueous solution method combined with subsequent calcination. The size of the precursor, FeSn(OH)6 cubes, was controlled carefully from 50–90 nm to 500–600 nm by adjusting the pH of the solution in the coprecipitation process. The structural and morphological evolution was characterized using a range of techniques. Thermal decomposition of the precursors led to an intimate mixture of hexagonal phase Fe2O3 and tetragonal phase SnO2. The as-prepared porous Fe2O3/SnO2 nanocubes with edge sizes in the range of 50–90 nm as anode materials for lithium storage achieved a very high reversible capacity of 620.8 mA h g−1 at 200 mA g−1 after 150 cycles. This paper reports a promising method for the design and preparation of porous nanocomposite electrodes for Li-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...