An access to 1,3-azasiline-fused quinolinones via oxidative heteroannulation involving silyl C(sp3)–H functionalization†
Abstract
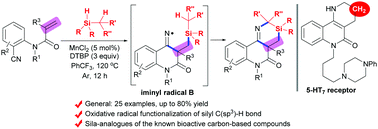
A Mn-promoted intermolecular oxidative radical heteroannulation of N-(2-cyanoaryl)-acrylamides and tertiary silanes has been described, which provides an efficient route to produce silicon/nitrogen heterocycles, sila-analogues of the known carbon-based structural motifs prevalent in bioactive natural products, pharmaceuticals and materials. The reaction enables Si-incorporation by controlling accurately several chemical bond cleavage and formation processes. Moreover, this reaction represents a new one-step construction of 1,3-azasiline-fused quinolinones that was achieved via silyl C(sp3)–H functionalization using an oxidative radical strategy.


 Please wait while we load your content...
Please wait while we load your content...