Aromatic aldehyde-selective aldol addition with aldehyde-derived silyl enol ethers†
Abstract
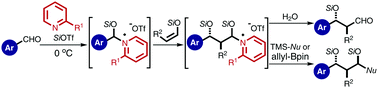
The aldol reaction using aldehyde-derived silyl enolates as nucleophiles with aromatic aldehydes chemoselectively proceeded in the presence of silyl triflate and 2,2′-bipyridyl to produce β-siloxy aldehydes, while the aliphatic aldehydes were completely recovered. The unprecedented chemoselectivities depend on the reactivities of the pyridinium-type intermediates derived from the aromatic and aliphatic aldehydes.


 Please wait while we load your content...
Please wait while we load your content...