Thiazolium salt-catalyzed C–C triple bond cleavage for accessing substituted 1-naphthols via benzannulation†
Abstract
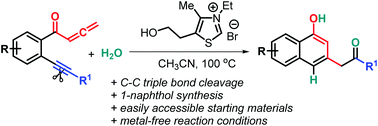
The first thiazolium salt-catalyzed C–C triple bond cleavage of benzene-linked allene-ynes has been established. The reaction pathway involves [2+2] cycloaddition and ring-opening of in situ generated cyclobutenes with H2O under mild and convenient conditions, and provides practical access to substituted 1-naphthols with potentially valuable applications.


 Please wait while we load your content...
Please wait while we load your content...