An acyclic zincagermylene: rapid activation of dihydrogen at sub-ambient temperature†
Abstract
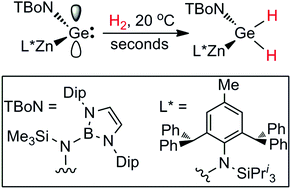
The first example of a stable zincagermylene, :Ge(TBoN)(ZnL*) (TBoN = N(SiMe3){B(DipNCH)2}, Dip = C6H3Pri2-2,6; L* = –N{C6H2[C(H)Ph2]2Me-2,6,4}(SiPri3)) is prepared and shown to have unprecedented reactivity for a germylene, with respect to the activation of dihydrogen. Computational analyses point towards this being partially derived from the electron releasing nature of the amido–zinc fragment, which leads to a narrowing of the HOMO–LUMO gap in the compound.


 Please wait while we load your content...
Please wait while we load your content...