Imidazole synthesis by transition metal free, base-mediated deaminative coupling of benzylamines and nitriles†
Abstract
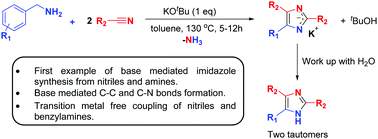
A transition metal free, straightforward synthetic method for the preparation of substituted imidazoles is reported herein. Base promoted, deaminative coupling of benzylamines with nitriles results in the one-step synthesis of 2,4,5-trisubstituted imidazoles with liberation of ammonia. This protocol provides a practical strategy for the synthesis of valuable imidazole derivatives from readily available starting materials.


 Please wait while we load your content...
Please wait while we load your content...