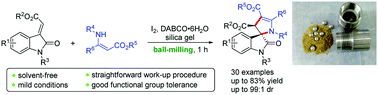
Solvent-free iodine-promoted synthesis of 3,2′-pyrrolinyl spirooxindoles from alkylidene oxindoles and enamino esters under ball-milling conditions†
Abstract
A novel solvent-free iodine-promoted cyclization of alkylidene oxindoles with enamino esters via C–C/C–N bond formation has been demonstrated under ball-milling conditions. This protocol provides efficient and green access to a variety of 3,2′-pyrrolinyl spirooxindoles with remarkable functional group tolerance, good yields and excellent diastereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...