Nickel-catalyzed amination of aryl fluorides with primary amines†
Abstract
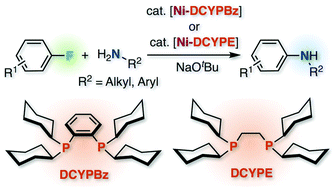
The Ni-catalyzed cross-coupling reaction between aryl fluorides and primary amines was enabled by the 1,2-bis(dicyclohexylphosphino)benzene (DCYPBz) or 1,2-bis(dicyclohexylphosphino)ethane (DCYPE) ligands. Both N-alkyl- and N-aryl-substituted primary amines participated in the selective reaction to form secondary amines. This protocol would potentially be useful for late-stage diversification of fluorinated compounds with complex structures for the synthesis of functionally interesting aniline derivatives.


 Please wait while we load your content...
Please wait while we load your content...