Iron-catalyzed peroxidation–carbamoylation of alkenes with hydroperoxides and formamides via formyl C(sp2)–H functionalization†
Abstract
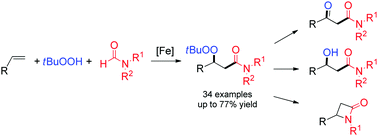
A reaction protocol in which FeCl3 and tert-butyl hydroperoxide facilitated a selective radical coupling reaction of aryl alkenes or 1,3-enynes with tert-butyl hydroperoxide and formamides to prepare an array of β-peroxy amides has been achieved. The β-peroxy amide could serve as a synthetic precursor which was facilely converted to β-hydroxy amide, β-keto amide and β-lactam following subsequent chemical transformation.


 Please wait while we load your content...
Please wait while we load your content...