Palladium-catalyzed denitrogenative functionalizations of benzotriazoles with alkenes and 1,3-dienes†
Abstract
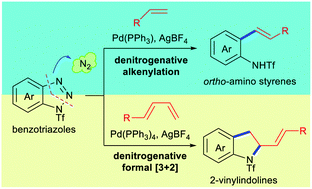
Pd-Catalyzed denitrogenative functionalizations of benzotriazoles with alkenes and 1,3-dienes have been developed, which enable the rapid access of diverse ortho-amino styrenes and 2-vinylindolines, respectively. This study shows the great potential of benzotriazoles as a [1C]-synthon in cross-coupling reactions and an aza-[3C]-synthon in cycloaddition reactions.


 Please wait while we load your content...
Please wait while we load your content...