Visible-light promoted γ-cyanoalkyl radical generation: three-component cyanopropylation/etherification of unactivated alkenes†
Abstract
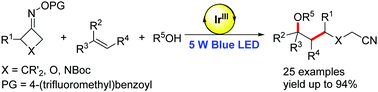
A photoredox approach to generate distal cyano-substituted alkyl radicals through C–C bond cleavage of cyclobutanone oximes was developed. The radicals produced by this method were applied to three-component cyanopropylation/etherification of unactivated alkenes. Their reactions with diverse radical acceptors were also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...