Boron–nitrogen main chain analogues of polystyrene: poly(B-aryl)aminoboranes via catalytic dehydrocoupling†
Abstract
The first high molar mass polyaminoboranes with an organic substituent at boron, namely the B-arylated polyaminoboranes [NH2–BHPh]n (2a) and [NH2–BH(p-CF3C6H4)]n (2b), have been prepared via catalytic dehydropolymerisation. These materials can be considered as inorganic analogues of polystyrene with a B–N main chain. Their synthesis was achieved from B-aryl amine–borane precursors in solution using an [IrH2(POCOP)] precatalyst.
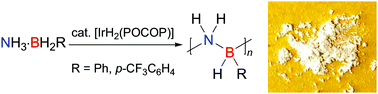


 Please wait while we load your content...
Please wait while we load your content...