Unprecedented rearrangement of diketopyrrolopyrroles leads to structurally unique chromophores†
Abstract
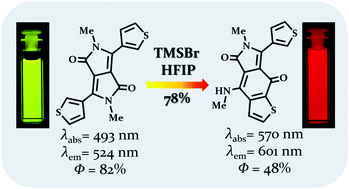
Diketopyrrolopyrroles possessing thienyl, furyl and benzofuryl substituents undergo unprecedented skeletal rearrangement in the presence of trimethylsilyl bromide resulting in the formation of thieno[2,3-f]isoindole-5,8-diones and furo[2,3-f]isoindole-5,8-diones. These relatively small dyes possess favorable photophysical properties with the emission maxima within the range of 573–624 nm, large fluorescence quantum yields, moderate sensitivity of emission to solvent polarity and a HOMO–LUMO gap of ca. 1.8 eV.


 Please wait while we load your content...
Please wait while we load your content...