N-Heterocyclic carbene-catalyzed sulfa-Michael addition of enals†
Abstract
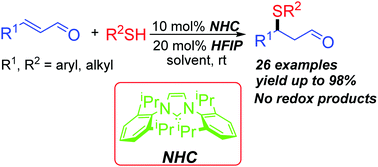
An efficient N-heterocyclic carbene (NHC) catalyzed sulfa-Michael addition (SMA) between enals and thiols has been developed. Under the catalysis of 10 mol% stable free carbene IPr and with 20 mol% hexafluoroisopropanol (HFIP) as an additive, enals react with a variety of thiols to afford the SMA adducts in 54–98% yields. In this process, the free carbene preferentially interacts with thiols through hydrogen-bonding and no NHC-catalyzed extended Umpolung transformations were observed.


 Please wait while we load your content...
Please wait while we load your content...