Diastereo- and enantioselective phase-transfer alkylation of 3-substituted oxindoles with racemic secondary alkyl halides†
Abstract
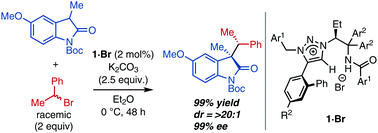
A catalytic asymmetric alkylation of fully substituted enolates with racemic, non-activated secondary alkyl halides is described. The chiral 1,2,3-triazolium ion enables excellent diastereo- and enantiocontrol via enantiofacial discrimination of prochiral enolates and kinetic resolution of secondary halides.


 Please wait while we load your content...
Please wait while we load your content...