Zirconium arene triple-decker sandwich complexes: synthesis, electronic structure and bonding†
Abstract
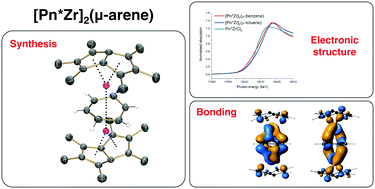
Reduction of a permethylpentalene zirconium(IV) chloride complex [η8-Pn*Zr(μ-Cl)3/2]2(μ-Cl)2Li·THFx with KC8 in benzene results in activation of the aromatic solvent to yield an “inverted sandwich” complex, [η8-Pn*Zr]2(μ-η6:η6-C6H6) (1). The reactions in toluene, cumene, o-xylene and m-xylene also yield analogous solvent activated triple-decker sandwich complexes, which have been structurally characterised by single-crystal X-ray diffraction. Edge energies in the Zr K-edge XANES spectra are not distinguishable between 1 and formally Zr(II) and Zr(IV) reference compounds, suggesting a broad edge structure. DFT calculations best describe the bonding in 1 as highly covalent with frontier molecular orbitals showing almost equal contributions from benzene and the Zr-permethylpentalene fragments.


 Please wait while we load your content...
Please wait while we load your content...