Nickel-catalyzed acetamidation and lactamization of arylboronic acids†
Abstract
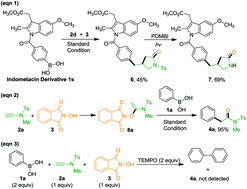
A nickel-catalyzed acetamidation and lactamization of arylboronic acids via a one-pot reaction with ynamides and N-hydroxyphthalimide is described. This protocol features with mild reaction conditions and a broad substrate scope, and has been successfully applied to late-stage functionalization of pharmaceuticals. Moreover, control reactions were conducted to elucidate a plausible mechanism.


 Please wait while we load your content...
Please wait while we load your content...