Copper(i)-catalysed asymmetric allylic reductions with hydrosilanes†
Abstract
A copper(I)-catalysed asymmetric allylic reduction enables a regio- and stereoselective transfer of a hydride nucleophile in an SN2′-fashion onto allylic bromides. This transformation represents a conceptually orthogonal approach to allylic substitution reactions with carbon nucleophiles. A copper(I) complex based upon a chiral N-heterocyclic carbene (NHC) ligand allows for stereoselectivity reaching 99% ee. The catalyst enables a stereoconvergent reaction irrespective of the double bond configuration of the starting materials.
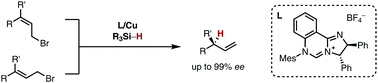


 Please wait while we load your content...
Please wait while we load your content...