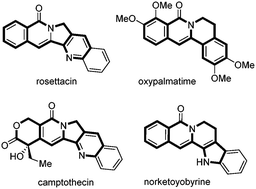
Rhodium(iii)-catalyzed intramolecular annulation through C–H activation: concise synthesis of rosettacin and oxypalmatime†
Abstract
A flexible and efficient rhodium(III)-catalyzed intramolecular annulation of benzamides bearing tethered alkynes for the synthesis of indolizinones and quinolizinones is reported. This reaction shows a broad substrate scope and excellent functional-group tolerance, including different kinds of heterocyclic substrates, such as furan, thiophene, pyrrole, benzofuran, benzothiophene, indole and isonicotinamide substrates. This method also provides a practical and efficient approach for the synthesis of rosettacin and oxypalmatime.


 Please wait while we load your content...
Please wait while we load your content...