Reversing the undesirable pH-profile of doxorubicin via activation of a di-substituted maleamic acid prodrug at tumor acidity†
Abstract
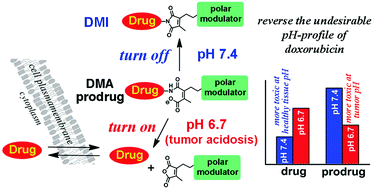
The acid-labile behavior of di-substituted maleamic acid (DMA) and its equilibrium with di-substituted maleimide (DMI) are exploited to build an ultra acid-sensitive, small molecule prodrug that can be activated by tumor extracellular pH (pHe) in the range of 6.5–6.9. Such a DMA prodrug reversed the unfavorable pH-profile of doxorubicin (Dox), which may improve its therapeutic window.


 Please wait while we load your content...
Please wait while we load your content...