Non-planar oligoarylene macrocycles from biphenyl†
Abstract
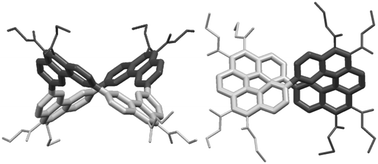
Saddle- and propeller-shaped macrocycles are obtained by fourfold Perkin condensations in transient high dilution of 4,4′-bis(phenylglyoxylic acid) with either 4,4′-bis(phenylacetic acid) or p-phenylenediacetic acid, followed by four-fold oxidative photocyclizations. In the saddle-shaped tetraphenanthrylene, the angle between opposite phenanthrene planes is close to the value of 90° of an ideal saddle. In the two helicene fragments of the propeller-shaped double [5]helicene, the geometry of unsubstituted [5]helicene is preserved without major macrocycle-induced distortions.


 Please wait while we load your content...
Please wait while we load your content...