Synthesis and shift-reagent-assisted full NMR assignment of bacterial (Z8,E2,ω)-undecaprenol†
Abstract
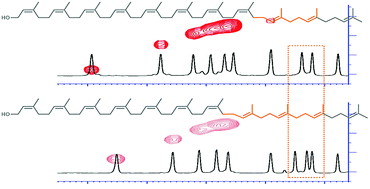
The repeating isoprene unit is a fundamental biosynthetic motif. The repetitive structure presents challenges both for synthesis and for structural characterization. In this synthesis of the (Z8,E2,ω)-undecaprenol of prokaryotic glycobiology, we exemplify solutions to these challenges. Allylation of sulfone-derived carbanions controlled the stereochemistry, and its proof-of-structure was secured by Eu(hfc)3 complexation to disperse the overlaid resonances of its 1H NMR spectrum.


 Please wait while we load your content...
Please wait while we load your content...