Palladium-catalyzed oxidative arylacetoxylation of alkenes: synthesis of indole and indoline derivatives†
Abstract
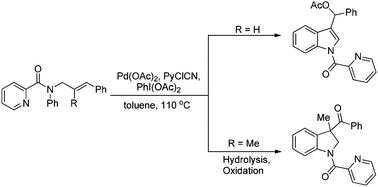
A method for the oxidative arylacetoxylation of alkenes has been developed to synthesize indole and indoline derivatives from readily accessible substrates. The cinnamyl tethered anilines with picolinamide as a directing group provided 3-substituted indoles via intramolecular oxidative arylacetoxylation, and the 2-methyl substituted cinnamyl anilines furnished indoline derivatives with 3-position quaternary stereocenters in good to excellent yields via sequential intramolecular oxidative arylacetoxylation, hydrolysis and oxidation steps.


 Please wait while we load your content...
Please wait while we load your content...