A vitamin B12 derivative catalyzed electrochemical trifluoromethylation and perfluoroalkylation of arenes and heteroarenes in organic media†
Abstract
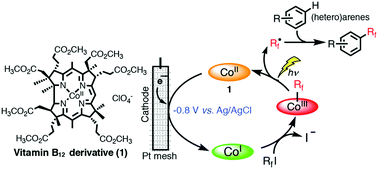
The electrochemical trifluoromethylation and perfluoroalkylation of aromatic compounds mediated by a vitamin B12 derivative as a cobalt-based catalyst has been developed. The Co(I) species of a vitamin B12 derivative, prepared by controlled-potential electrolysis at −0.8 V vs. Ag/AgCl in methanol, reacted with RfI (Rf = CF3, n-C3F7, n-C4F9, n-C8F17, and n-C10F21) to form a Co–Rf complex. This complex released an Rf radical under visible light irradiation, which then reacted directly with non-activated (hetero)arenes to form the desired fluoroalkylated molecules through direct C–H functionalization. To our knowledge, this is the first report of a naturally derived vitamin B12 catalyzed trifluoromethylation and perfluoroalkylation of aromatic compounds as a cobalt-mediated catalyst.


 Please wait while we load your content...
Please wait while we load your content...