An approach to spirooxindoles via palladium-catalyzed remote C–H activation and dual alkylation with CH2Br2†
Abstract
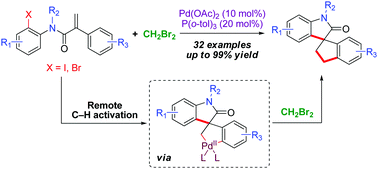
A facile and efficient approach for the synthesis of spirooxindoles has been developed via the coupling of spirocyclic C,C-palladacycles with CH2Br2. The key spirocyclic palladacycles are generated catalytically via remote C–H activation. A range of spirooxindoles can be synthesized in good to excellent yields from readily available starting material.


 Please wait while we load your content...
Please wait while we load your content...