A highly enantioselective asymmetric Darzens reaction catalysed by proline based efficient organocatalysts for the synthesis of di- and tri-substituted epoxides†
Abstract
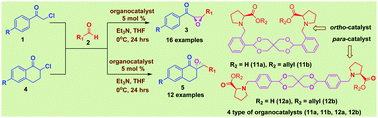
A new class of easily available and readily tunable proline based chiral organocatalysts was found to efficiently catalyse an unprecedented highly enantioselective asymmetric Darzens reaction of α-chloroketones and substituted α-chloroketones with various aldehydes, which directly produces optically active di- and tri-substituted chiral epoxides with higher product yields (up to 97%) and excellent ee's (up to 99%) under mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...