Pd–PEPPSI: a general Pd–NHC precatalyst for Buchwald–Hartwig cross-coupling of esters and amides (transamidation) under the same reaction conditions†
Abstract
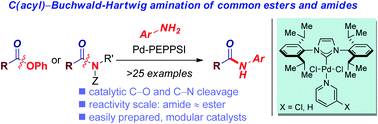
Amides are of fundamental interest in many fields of chemistry involving organic synthesis, chemical biology and biochemistry. Here, we report the first catalytic Buchwald–Hartwig coupling of both common esters and amides by highly selective C(acyl)–X (X = O, N) cleavage to rapidly access aryl amide functionality via a cross-coupling strategy. Reactions are promoted by versatile, easily prepared, well-defined Pd–PEPPSI type precatalysts, and proceed in good to excellent yields and with excellent chemoselectivity for the acyl bond cleavage. The method is user friendly because it employs commercially-available, moisture- and air-stable precatalysts. Notably, for the first time we demonstrate selective C(acyl)–N and C(acyl)–O cleavage/Buchwald–Hartwig amination under the same reaction conditions, which allows for streamlining amide synthesis by avoiding restriction to a particular acyl metal precursor. Of broad interest, this study opens the door to using a family of well-defined Pd(II)–NHC precatalysts bearing pyridine “throw-away” ligands for the selective C(acyl)-amination of bench-stable carboxylic acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...