Switching enzyme specificity from phosphate to resveratrol glucosylation†
Abstract
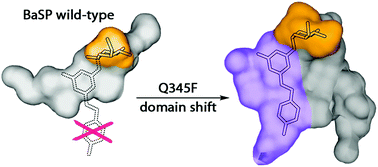
Here we present a point mutation-triggered domain shift which switches the acceptor preference of a sucrose phosphorylase from phosphate to a variety of large polyphenolic compounds including resveratrol and quercetin, enabling their efficient glucosylation. The variant possesses a high affinity for aromatic substrates due to newly introduced π–π- and hydrophobic interactions in the altered active site. The domain shift brings about a substantially enlarged and multifunctional active site for polyphenol glucosylation and rare disaccharide production. The crystal structure of the variant with its product resveratrol-3-α-D-glucoside allows the prediction of the substrate scope and regioselectivity of the aromatic compounds’ glucosylation sites.


 Please wait while we load your content...
Please wait while we load your content...