Photoredox catalysis enabled alkylation of alkenyl carboxylic acids with N-(acyloxy)phthalimide via dual decarboxylation†
Abstract
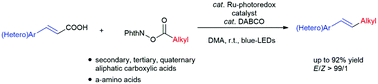
A ruthenium based photoredox catalyst in combination with a substoichiometric amount of 1,4-diazabicyclo[2.2.2]octane (DABCO) efficiently catalyzed dual decarboxylative couplings between alkenyl carboxylic acids and N-(acyloxy)phthalimides derived from aliphatic carboxylic acids, delivering alkylated styrene derivatives in a high regio- and stereo-selective manner under mild reaction conditions. Various types of secondary, tertiary, and quaternary aliphatic carboxylic acids as well as α-amino acids can be used as suitable substrates. Mechanistic analysis suggested that the reaction proceeds through a radical mechanism mediated by a Ru(I)/Ru(II) catalytic cycle with DABCO acting both as the base and the co-catalyst for single electron transfer.


 Please wait while we load your content...
Please wait while we load your content...