Scavenging activity of Magnéli phases as a function of Ti4+/Ti3+ ratios†
Abstract
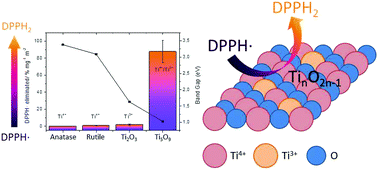
TiO2 is able to scavenge reactive oxygen and nitrogen species (ROS and RNS) in the absence of light. The scavenging mechanism has been related to the chemistry of defects (oxygen vacancy reduced oxidation states of Ti) but it is still unknown. This study describes the ROS scavenging activity of different titanium oxide phases and relates their scavenging activities with the Ti4+/Ti3+ molar ratio as well as the band gap value. The Ti5O9 phase, with a mixture of both oxidation states, presented a substantially higher percentage of 2,2-diphenyl-1-picrylhydracyl radicals (DPPH˙) eliminated per m2 of specific surface area in comparison to phases with predominant oxidation states Ti4+ or Ti3+ such as TiO2 and Ti2O3, respectively. The obtained results indicate that the DPPH˙ scavenging mechanism corresponds to a catalytic process on the Ti5O9 surface which is facilitated by the presence of charges that can easily move through the material. The mobility of charges and electrons in the semiconductor surface, related to the presence of oxidation states Ti4+ and Ti3+ and a small band gap, could create an attractive surface for radical species such as DPPH˙. This puts forward Ti5O9 as a promising candidate coating for implantable biomedical devices, as an electrode, since it can cushion inflammatory processes which could lead to device encapsulation and, consequently, failure.


 Please wait while we load your content...
Please wait while we load your content...