Light-promoted metal-free cross dehydrogenative couplings on ethers mediated by NFSI: reactivity and mechanistic studies†
Abstract
Cross dehydrogenative couplings on ethers occur very effectively using N-fluorobis(phenyl)sulfonimide (NFSI) as oxidizing agent under UVA irradiation in the presence of 2 mol% benzophenone. The reaction was shown to proceed first by fast radical fluorination of the α-C–H bond of ethers, followed by HF elimination to yield the highly electrophilic oxocarbenium ion as a key intermediate.
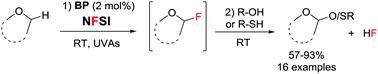


 Please wait while we load your content...
Please wait while we load your content...