Oxygenation of RZn(N,O)-type complexes as an efficient route to zinc alkoxides not accessible via the classical alcoholysis path†
Abstract
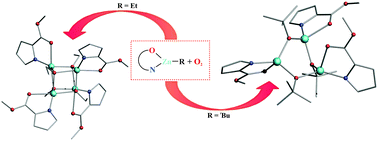
The controlled oxygenation of alkylzinc complexes supported by a 2-ester substituted pyrrolate ligand (L) leads to zinc alkoxides with an uncommon structural motif in the solid state: a trimer [(L)Zn(μ-OtBu)]3 with the central [Zn3(μ-OR)3] ring and a tetramer [(L)Zn(μ3-OEt)]4 with a heterocubane-type structure. Strikingly, these seemingly simple zinc alkoxides are not accessible via the classical alcoholysis route.


 Please wait while we load your content...
Please wait while we load your content...