Direct diversification of unmasked quinazolin-4(3H)-ones through orthogonal reactivity modulation†
Abstract
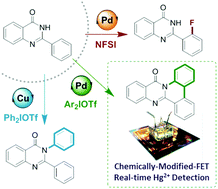
Here we report a set of direct functionalization methods of unmasked 2-phenylquinazolin-4(3H)-ones, a privileged alkaloid core, without the installation/removal event of protecting groups or exogenous coordinating moieties. Divergent pathways were modulated with transition-metal catalysts by suppressing competitive reactivities, leading to N-arylation, annulative π-extension, or C–H fluorination.


 Please wait while we load your content...
Please wait while we load your content...