Asymmetric conjugate addition of alkylzirconium reagents to α,β-unsaturated thioesters: access to fragrances and acyclic stereochemical arrays†
Abstract
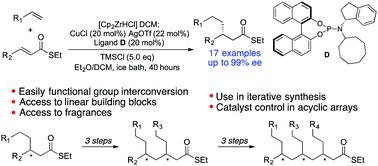
Copper-catalyzed asymmetric conjugate addition of alkylzirconium species to α,β-unsaturated thioesters is reported. A variety of functionalized alkyl nucleophiles were introduced with yields around 70% and ee's over 92%. The method was applied to the straightforward syntheses of the commercially important fragrances phenoxanol (both enantiomers 97% ee), and hydroxycitronellal (98% ee). The 1,4-addition products can be converted to enantiomerically enriched linear building blocks bearing a terminal functional group. Formation of further α,β-unsaturated thioesters provides an iterative route for the stereocontrolled synthesis of functionalized acyclic arrays and we demonstrate almost complete catalyst control in the formation of additional stereocentres.


 Please wait while we load your content...
Please wait while we load your content...